多功能植物光合表型成像测量系统
多功能植物光合表型成像测量系统PlantExplorer PRO+基于先进的叶绿素荧光成像技术和机器视觉技术,将植物表型参数和光合参数进行可视化、标准化和数字化。该设备集光合生理测量、可见光表型测量、色素测量和光谱指数测量于一身,用于植物光合作用机理、植物表型、植物胁迫生理、植物病理、基因型筛选、遗传育种、突变株筛选、胁迫损伤的早期检测、种子生理学、种子病理学等研究领域。
该系统采用最新的机器视觉技术和叶绿素荧光成像技术,将调制与非调制叶绿素荧光成像测量功能集于一身,结合最新的LED技术、CMOS技术、自动滤波轮技术及图像处理技术,并配备强大且友好的图形化界面用于操作控制和数据分析,实现对植物光合生理表型和形态结构表型的同步测量。其独特的成像舱体设计,既能保证系统的成像面积(53cm*53cm@D=60cm,D为植物冠层距离相机的距离),实现中大型冠幅植物的活体测量或全生育期测量;又确保测量过程各类光(光化光、饱和脉冲光等)的均一性与异质性,保证系统的成像质量与数据可靠性。
多功能植物光合表型成像测量系统PlantExplorer PRO+可根据用户需求,定制化增加/选配GFP成像与RFP成像模块(需在首次购买时指出,无法后续升级)。
详情请咨询:info@phenotrait.com

功能特性
- 创新的多功能植物表型平台(生理表型与形态表型)
- 可见光成像+多光谱成像+叶绿素荧光(调制和非调制)成像
- 同一相机获取所有成像结果
- 全自动马达聚焦系统,带全景和微距聚焦程序
- 出色的高清相机
- 高信噪比叶绿素荧光成像结果
- 大景深设计
- 自动计算荧光参数和表型参数
- 可设置进行延时成像测量
- 嵌入式电脑进行精确的成像、时间控制、光强控制和数据存储
- 系统配置触摸屏显示器
- 功能强大的控制和分析软件,内置分析算法
分析软件

主要技术参数
- 相机传感器类型:CMOS
- 相机分辨率:500万像素或1200万像素
- 曝光时间:1µs/step,从28µs到90 ms
- 图像格式:16位RAW格式
- 光谱范围:350~1000 nm
- 饱和光:450nm蓝光,6000μmol m-2 s-1@D=40cm(D为距离LED阵列以下的距离),强度可调
- Kautsky测量光:450nm蓝光,6000μmol m-2 s-1@D=40cm(D为距离LED阵列以下的距离),强度可调
- 光化光:宽带白色(3000K)、红色(660nm)、蓝色(450 nm),100-600μmol m-2 s-1@D=60cm(D为距离LED阵列以下的距离),强度可控
- 光谱波段:NIR(769nm)、Fluorescence(732nm)、Red Edge(710nm)、RED(640nm)、GREEN(550nm)、ANTH.(540nm)、BLUE(475nm)
- 光学滤光片:7种高质量光学干涉滤光片
- 成像面积:53cm × 53cm@D=60cm,(D为植株冠层距离相机镜头的距离)
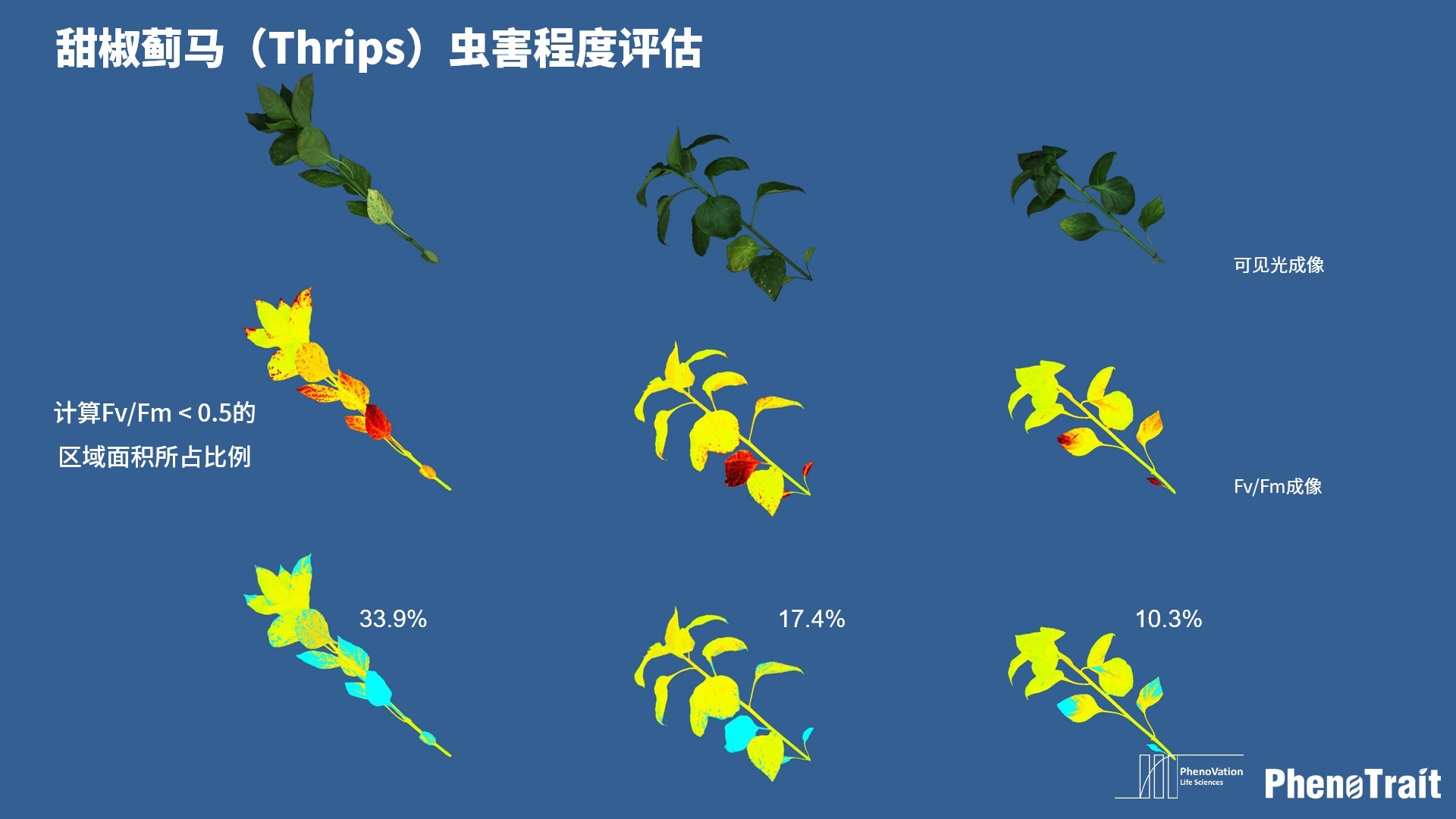
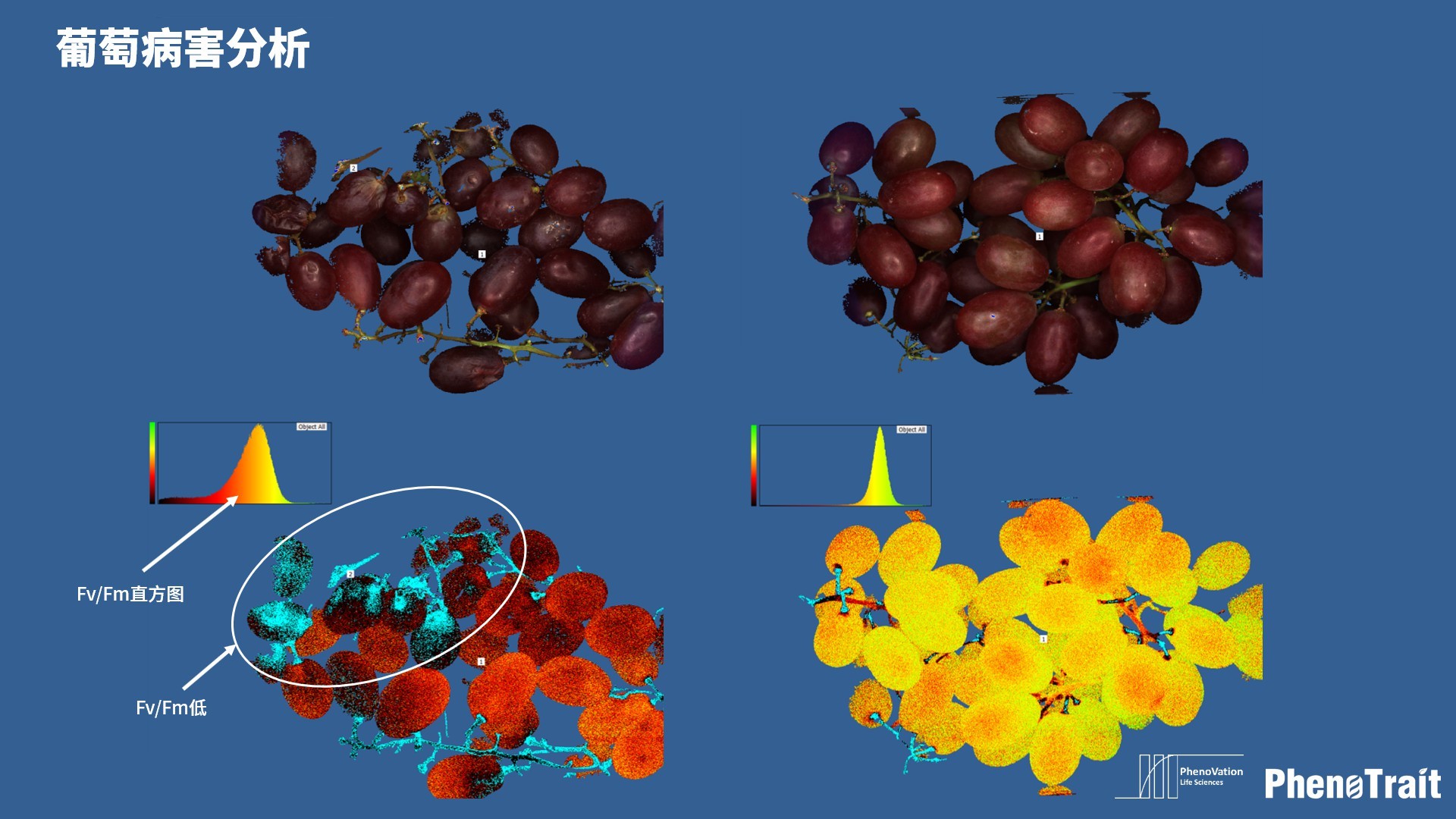
- 成像和计算的参数:Fo成像、Fm成像、Ft成像、Ft=5min成像、Fm’成像、Fv/Fm成像、Fq’成像、ΦPSII成像、ΦRO成像、NPQ100成像、qN成像、qP成像、Rfd100成像、 NDVI成像、RNIR成像、RChl成像.、RAnth成像、RRed成像、RGreen成像、RBlue成像、叶绿素指数成像、花青素指数成像和可见光成像,能够自动计算投影叶面积、Fv/Fm平均值、低于Fv/Fm的面积百分比、ΦPSII平均值、低于ΦPSII的面积百分比、NPQ100平均值、高于NPQ100的面积百分比、Rfd100平均值、低于Rfd100的面积百分比、平均RGB比值、特殊RGB比值的面积百分比、平均叶绿素指数、低于叶绿素指数的面积百分比、平均花青素指数、低于花青素指数的面积百分比等(具体参数取决于版本),以及凸包、最小外接圆、最小外接矩形等相关表型参数。
成像结果
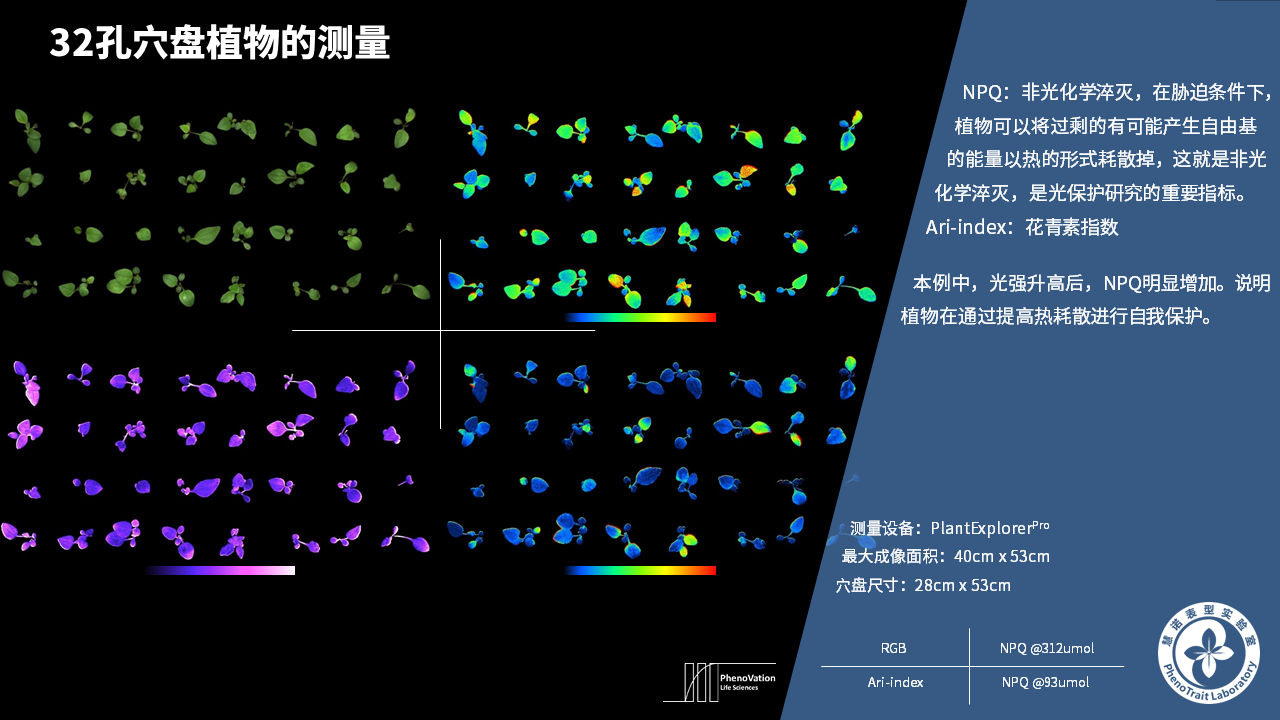
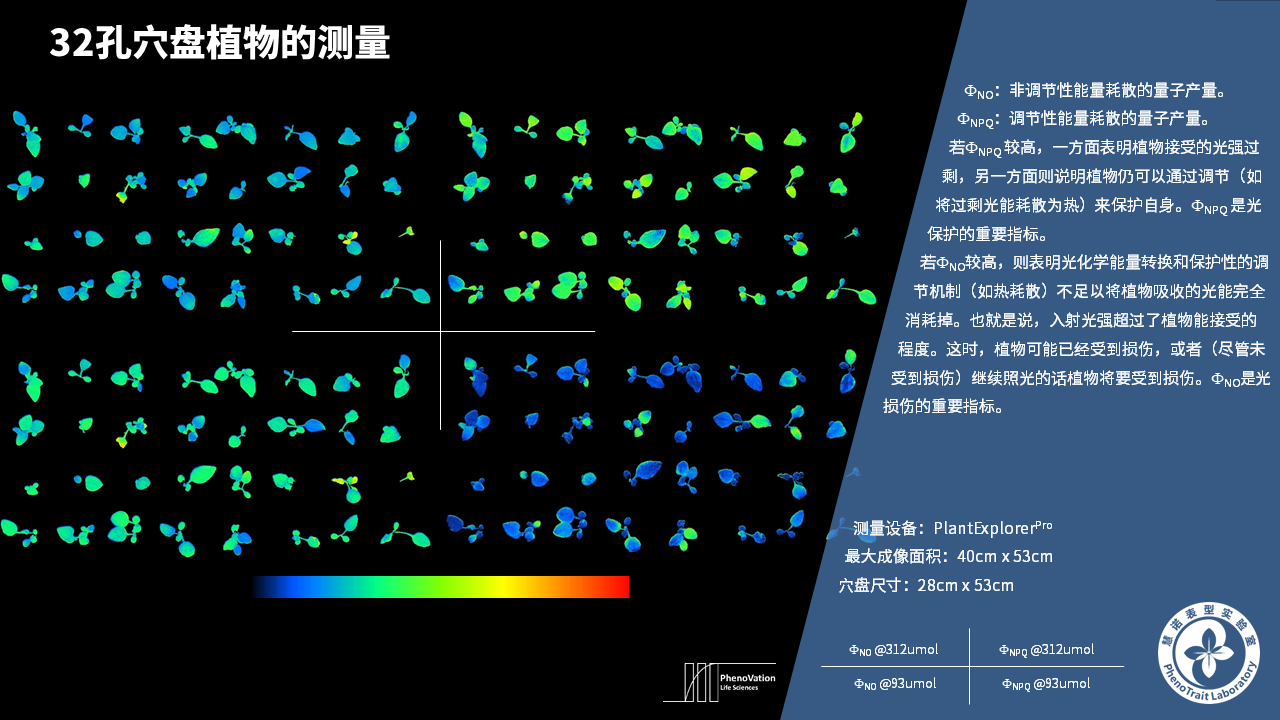
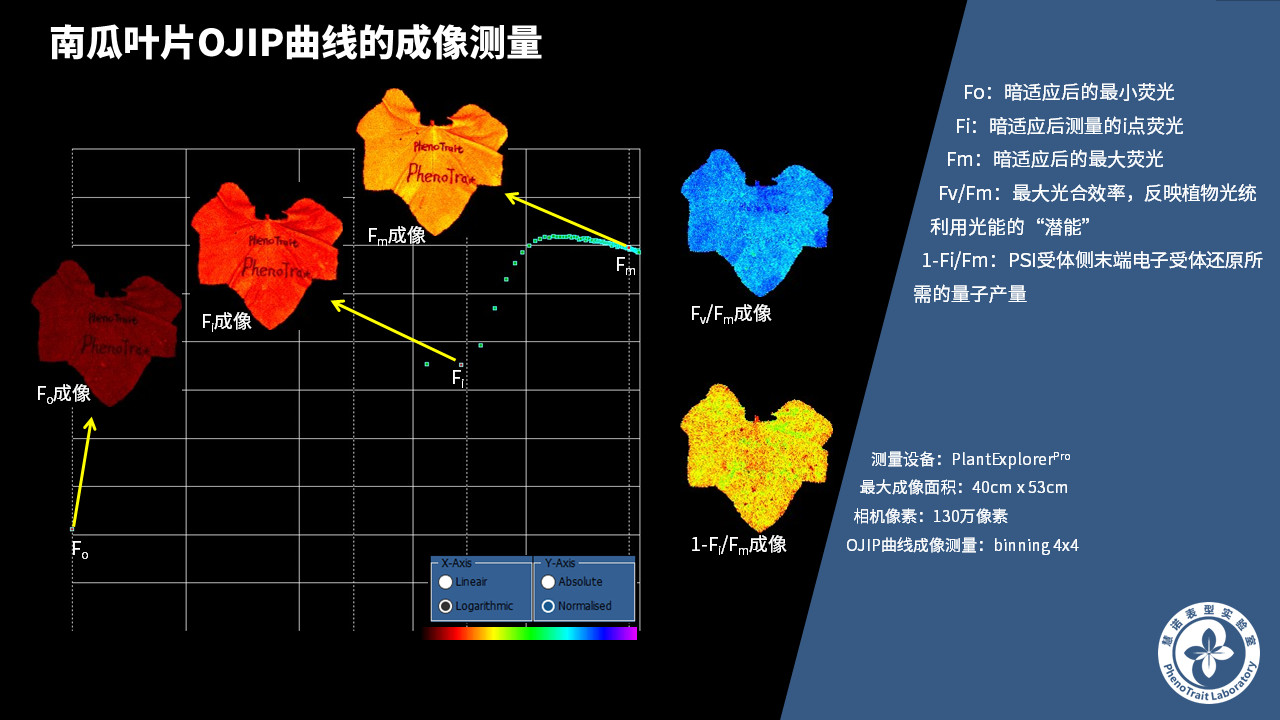

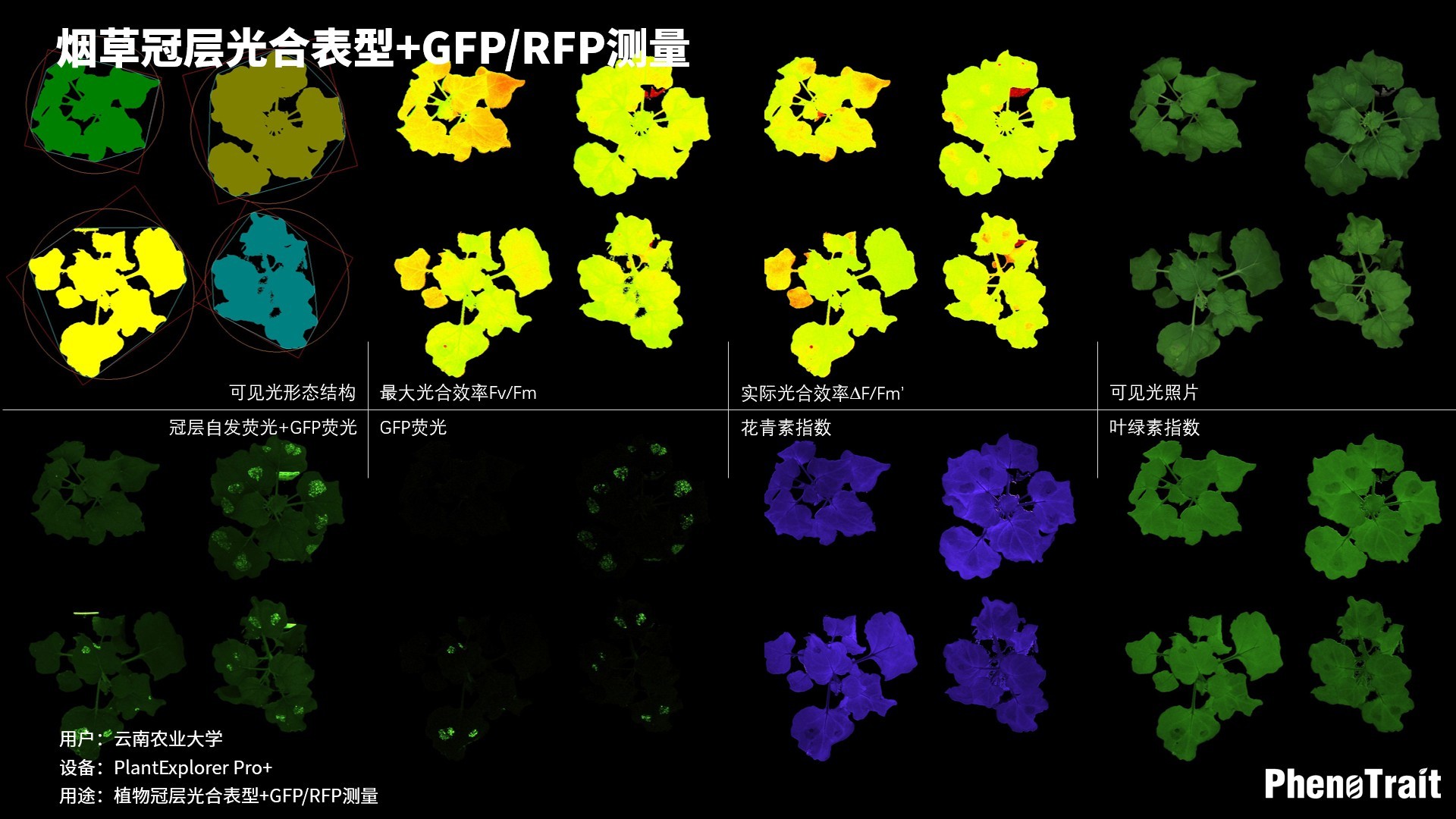


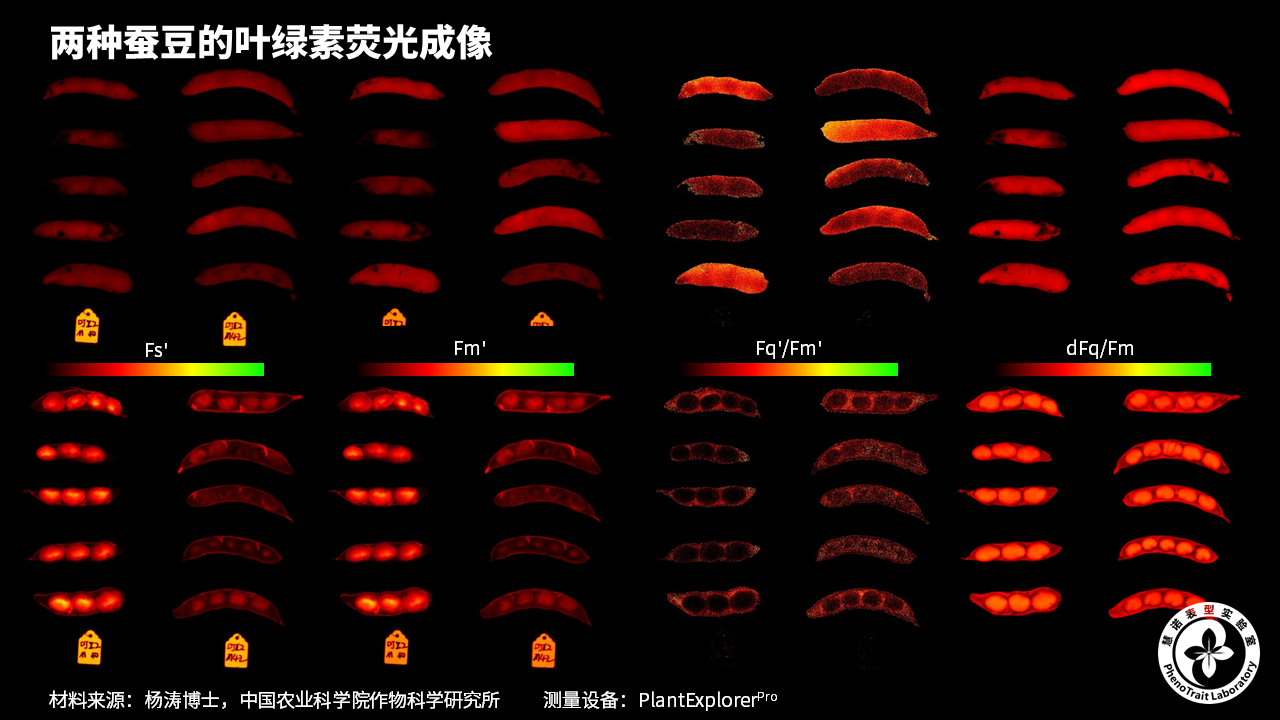
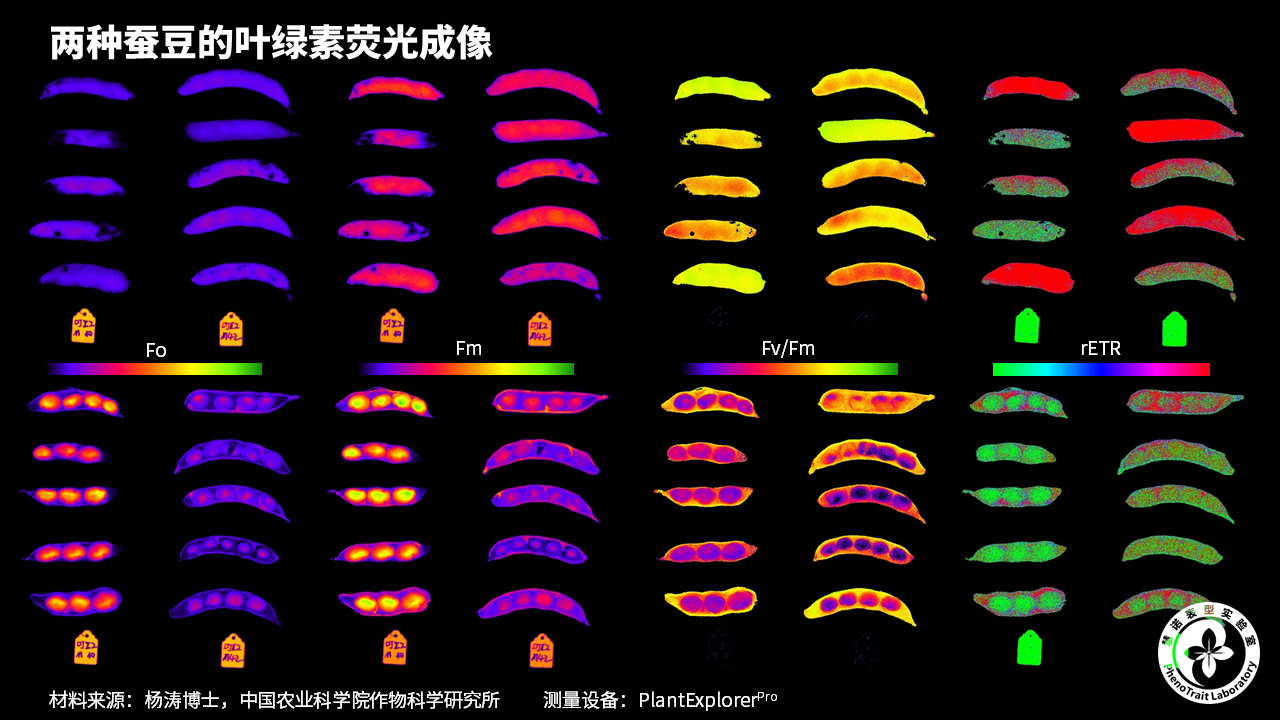
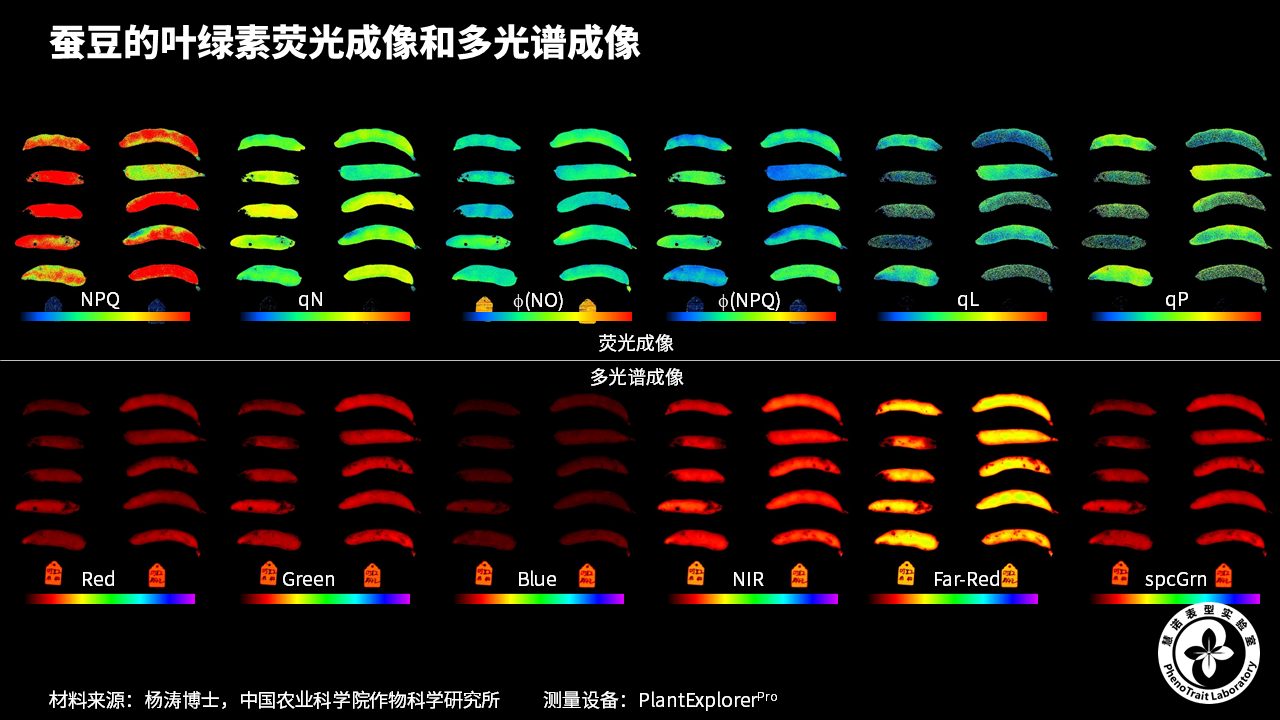
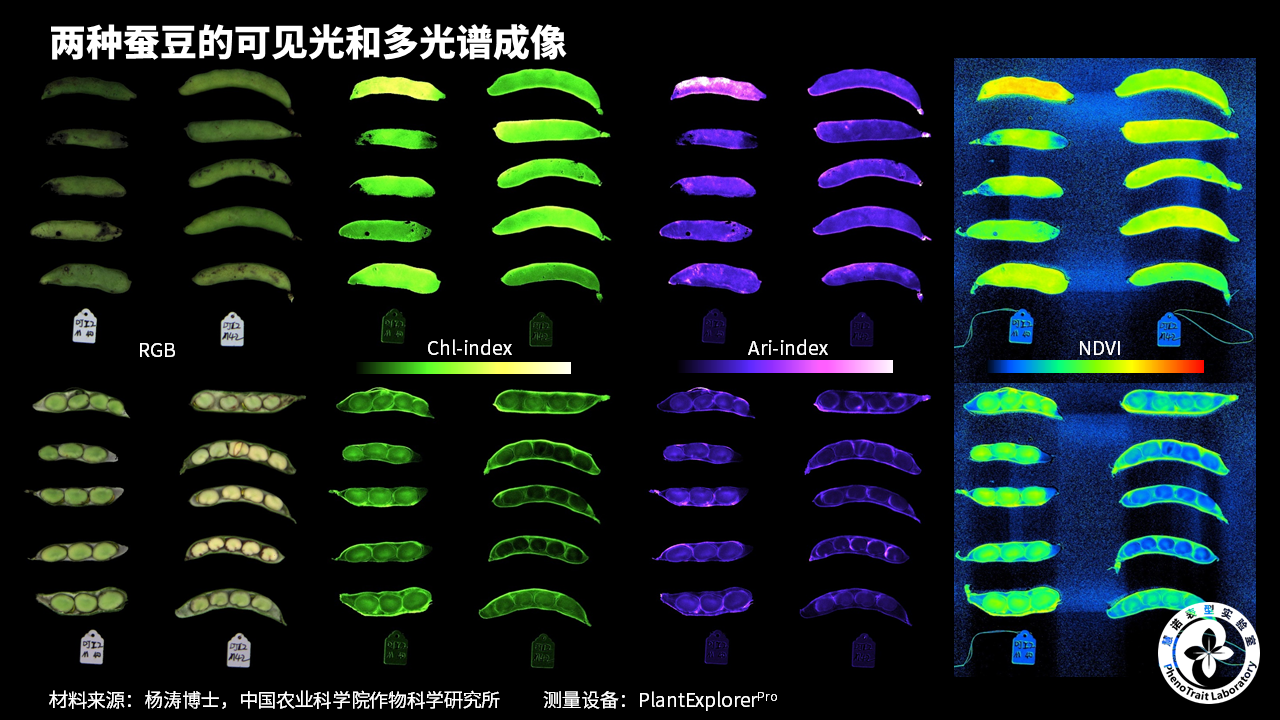
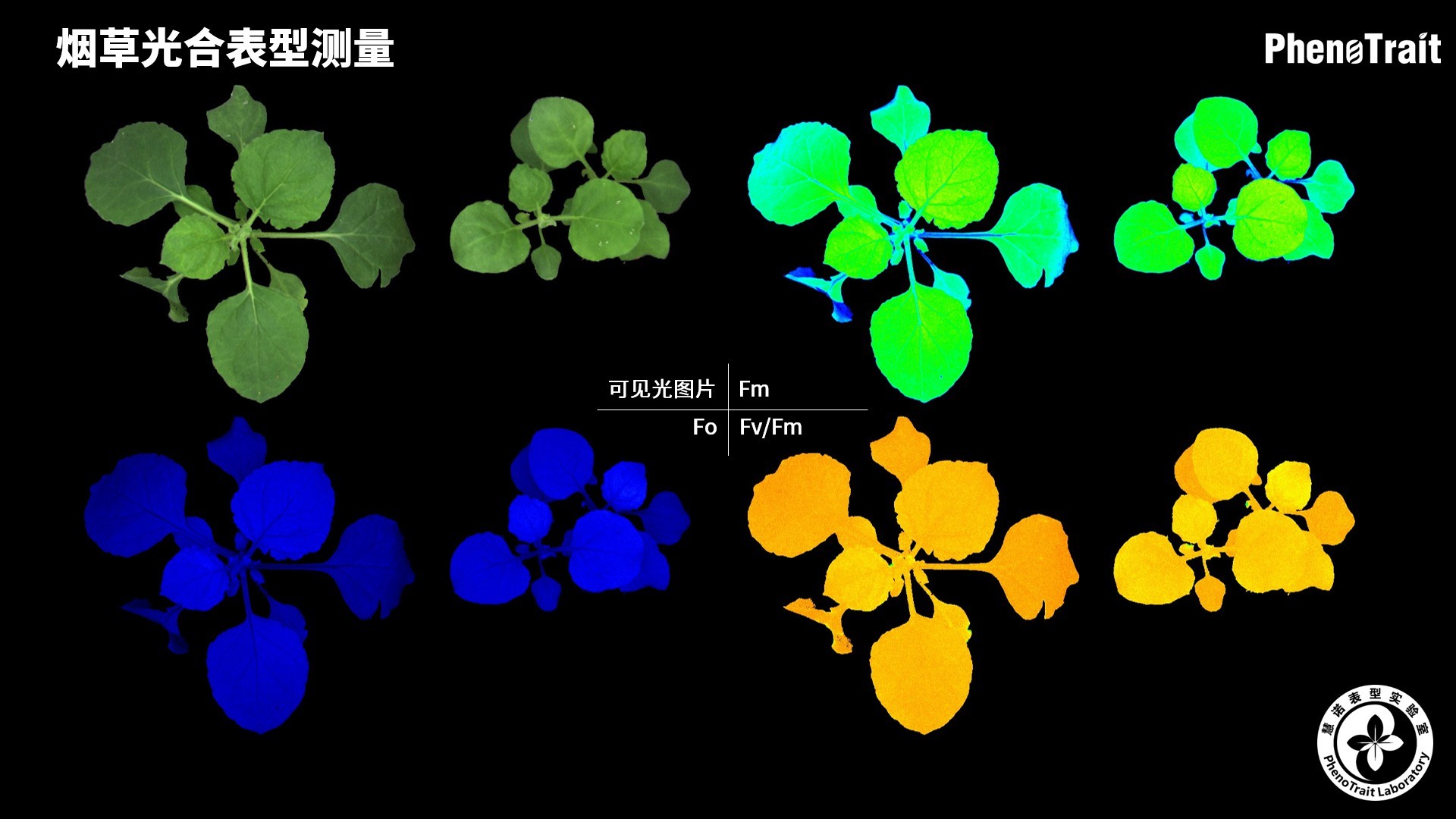

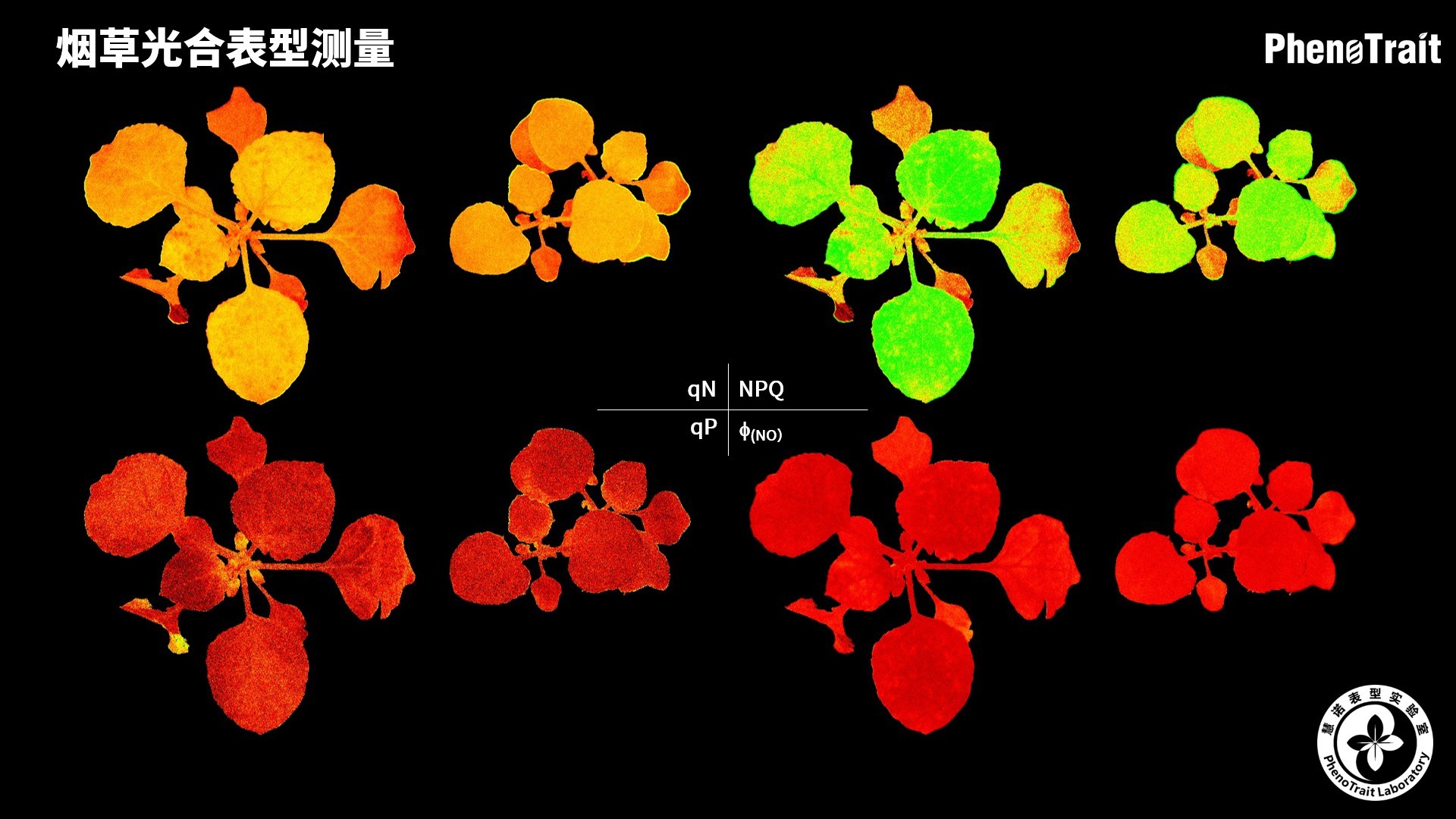

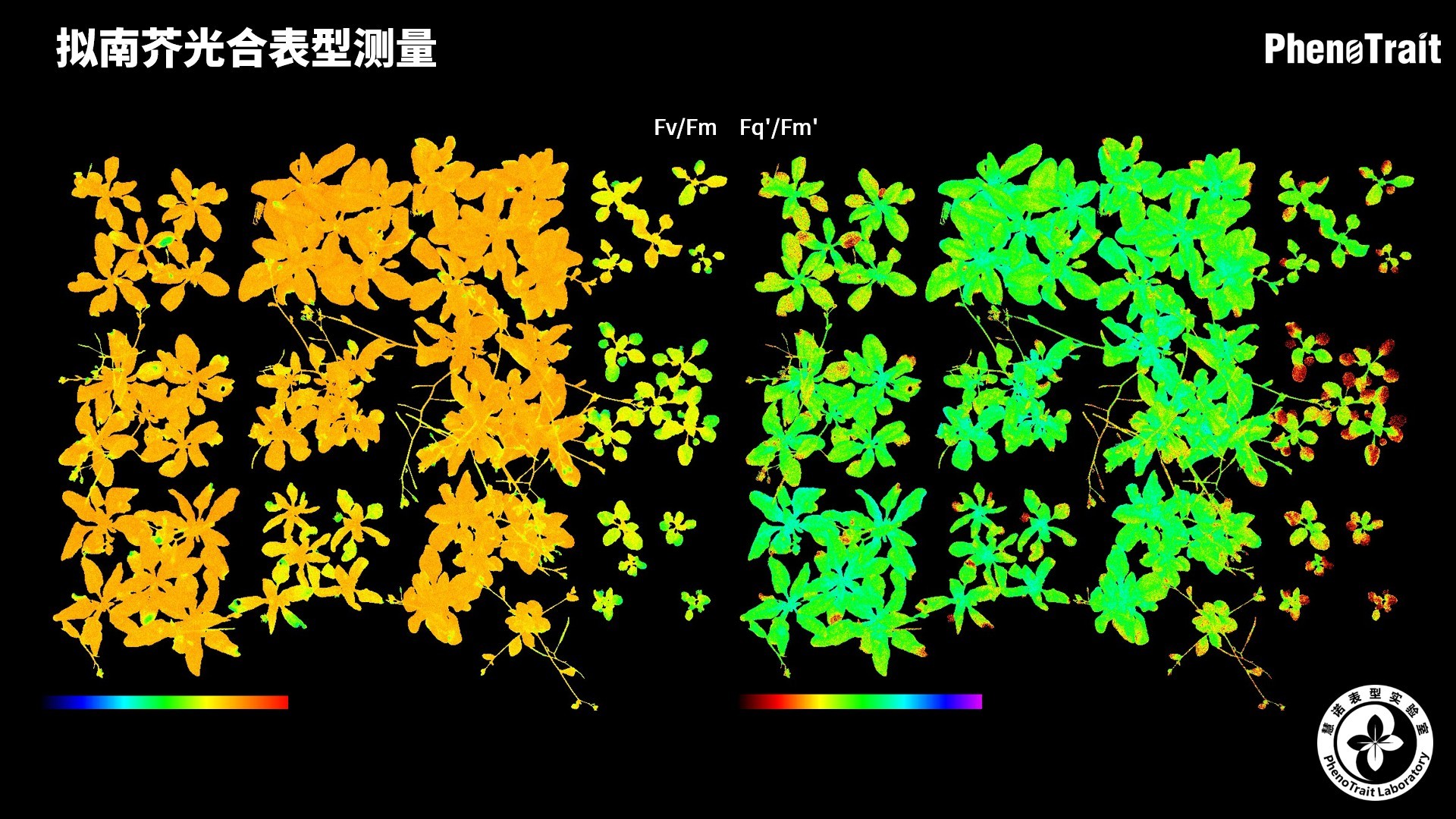
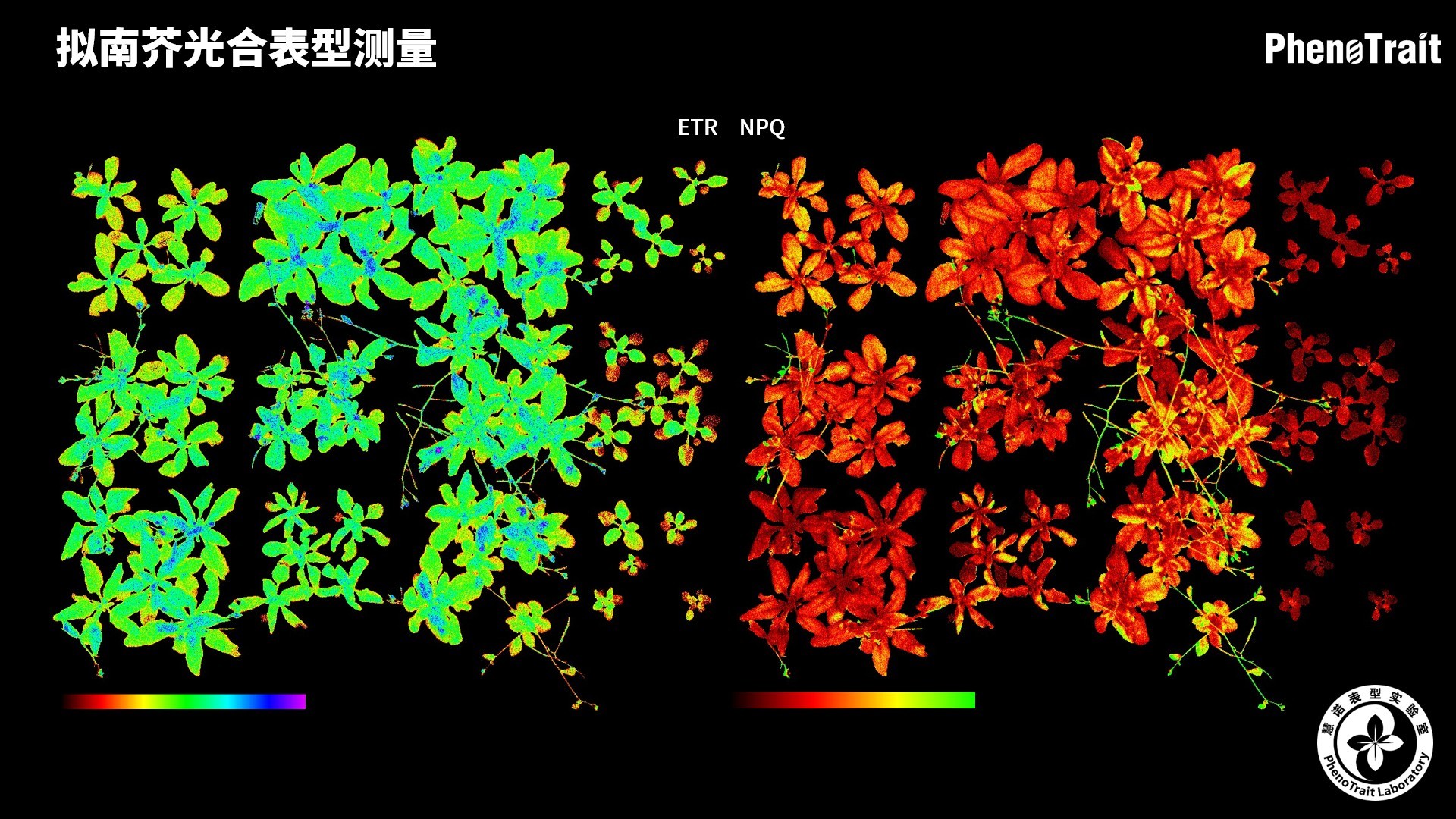
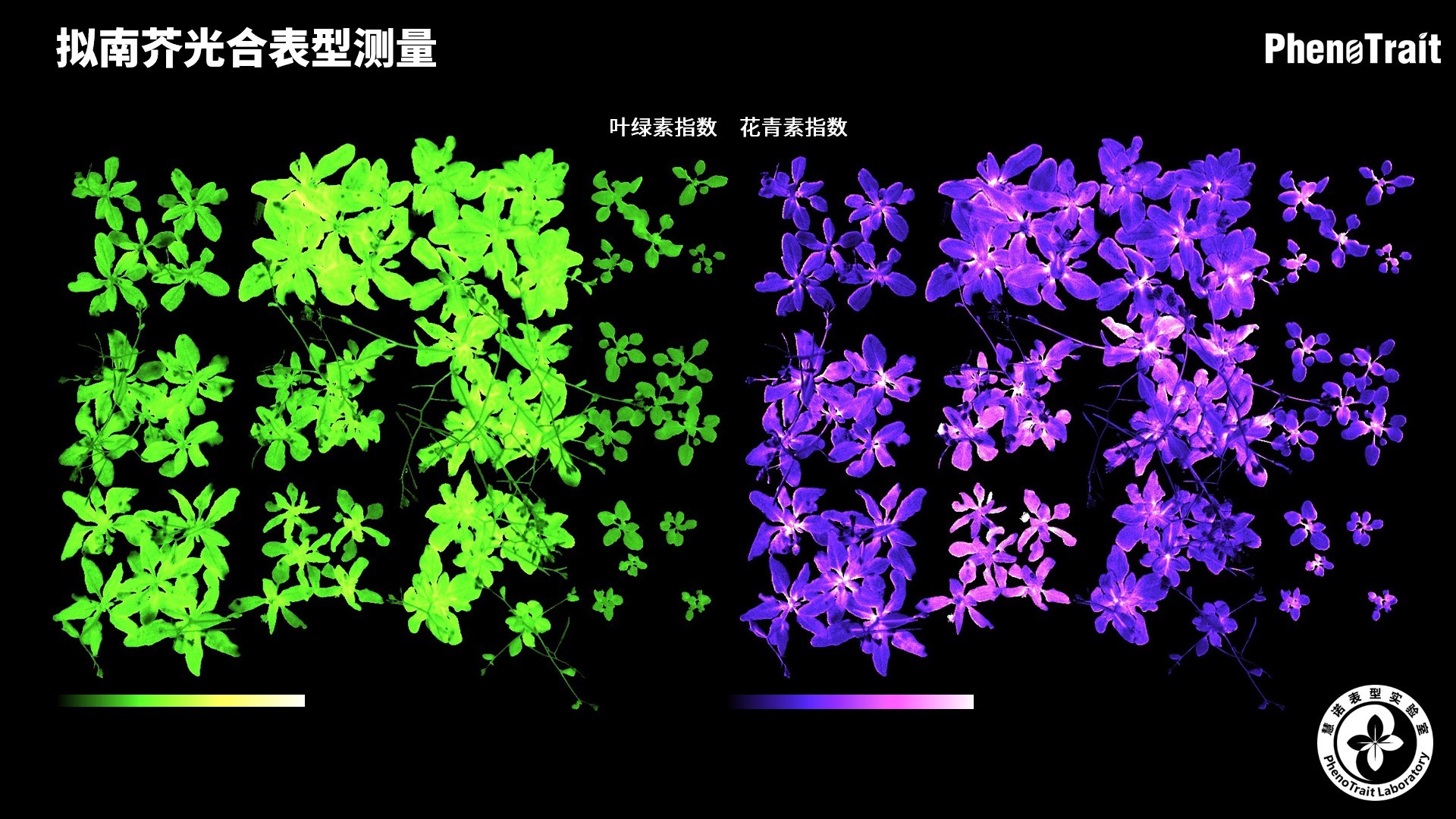
应用案例
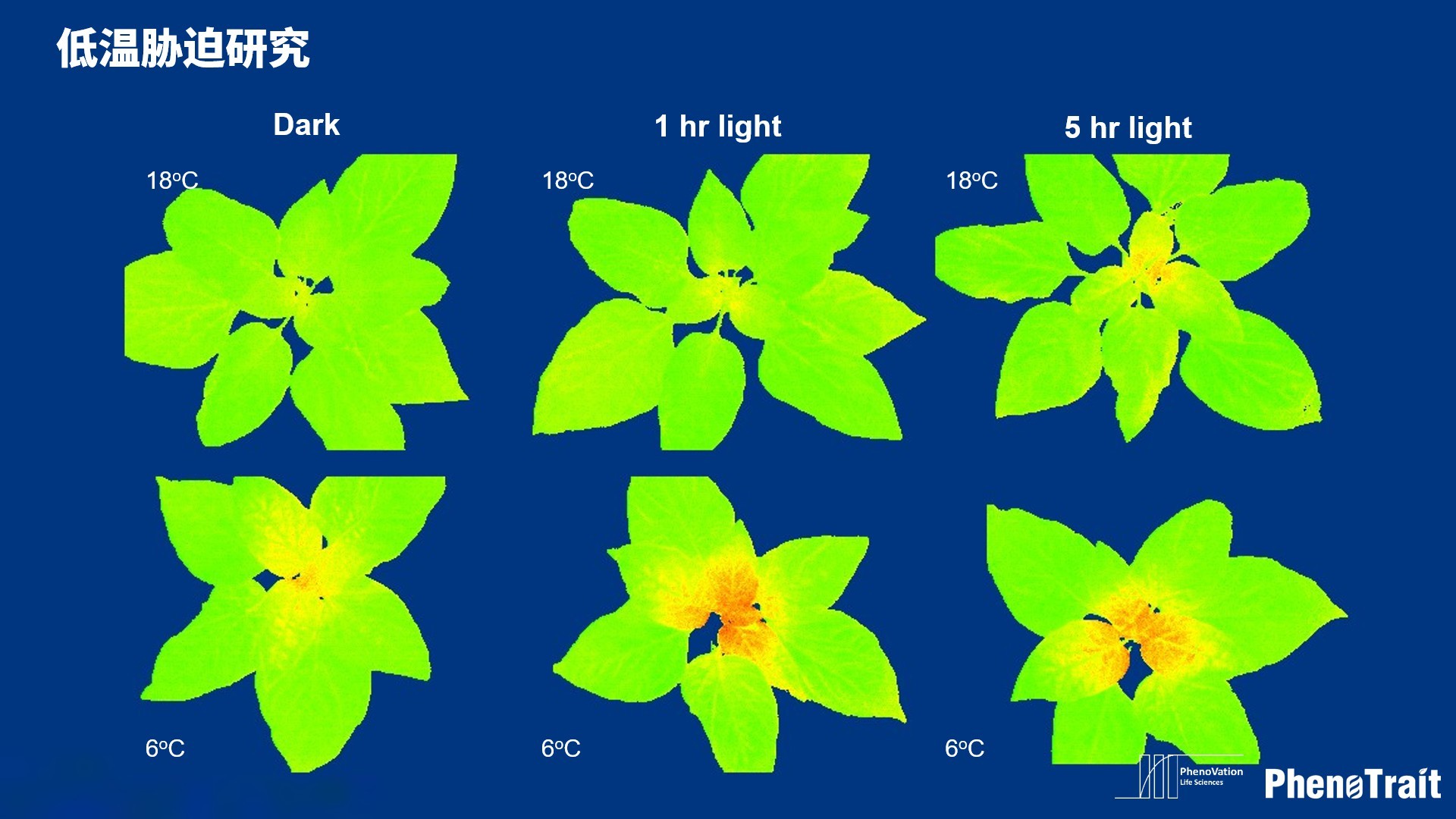
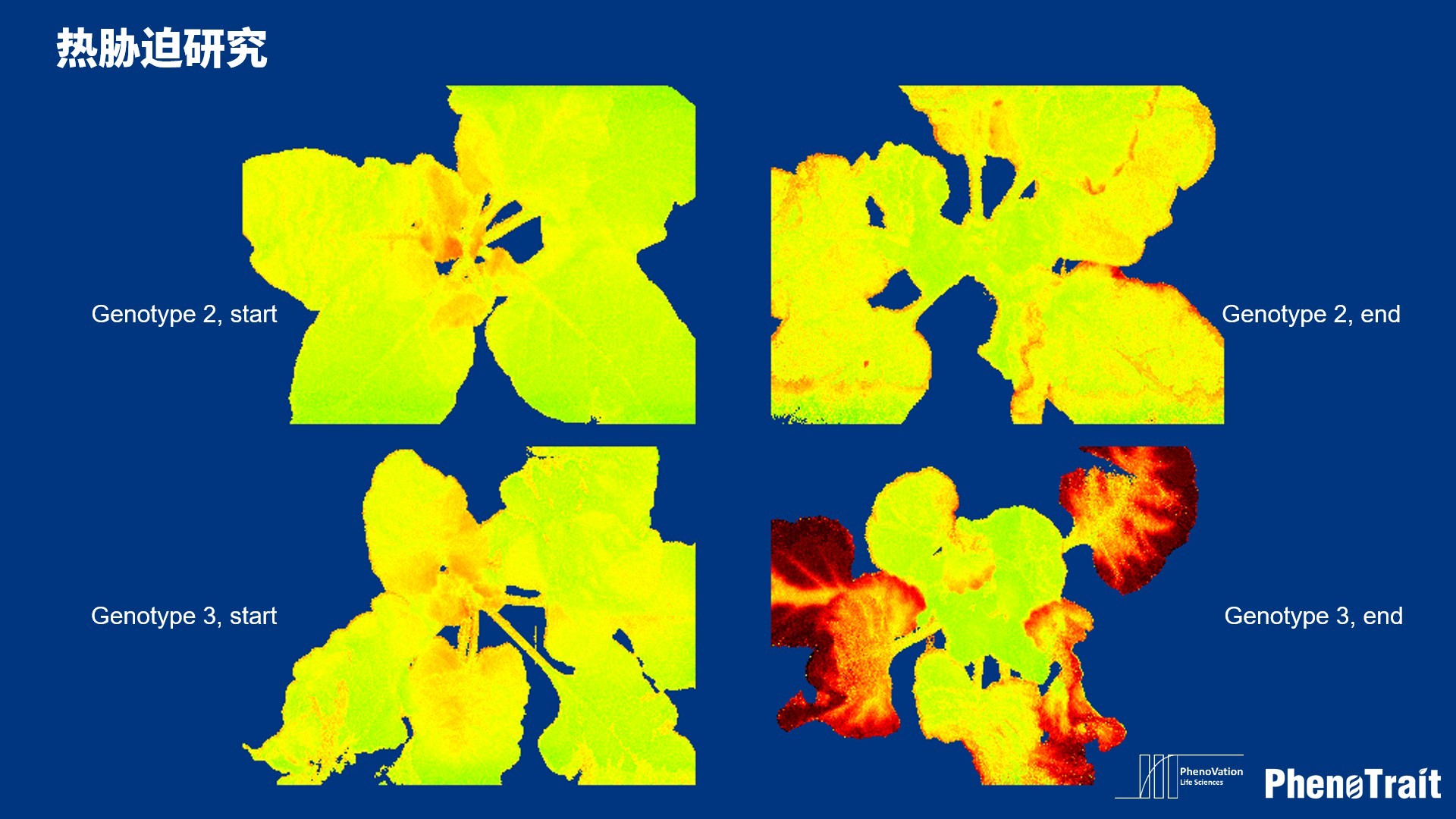
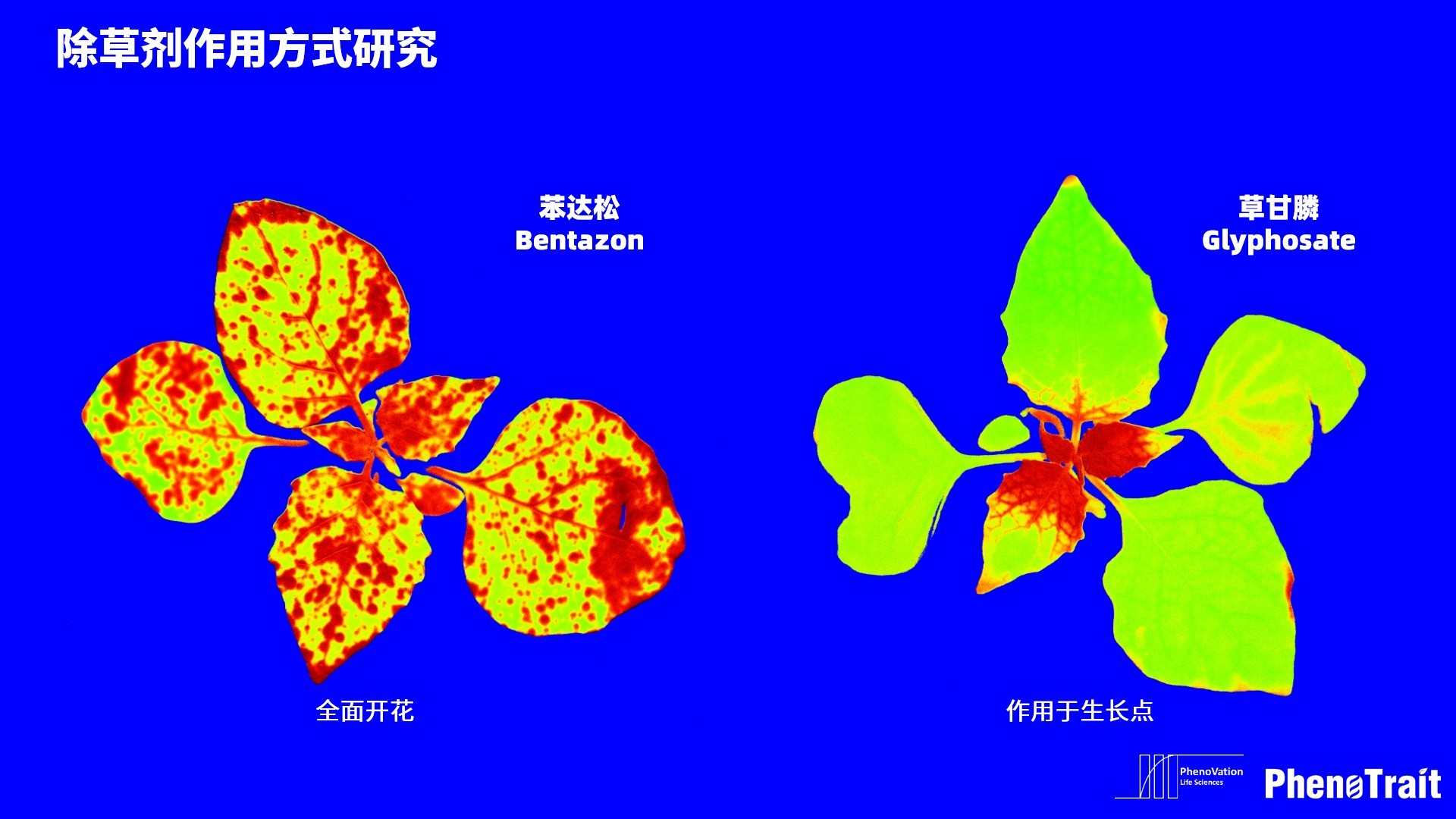


利用PhenoVation光合表型成像技术发表的部分文献
- Casto A L, Schuhl H, Schneider D, et al. (2021) Analyzing chlorophyll fluorescence images in PlantCV. Earth and Space Science Open Archive:5. https://doi.org/10.1002/essoar.10508322.2
- Wang L, Liu F, Hao X, et al. (2021) Identification of the QTL-allele System Underlying Two High-Throughput Physiological Traits in the Chinese Soybean Germplasm Population. Frontiers in Genetics, https://doi.org/10.3389/fgene.2021.600444
- Farooq M, van Dijk A D J, Nijveen H, et al. (2021) Prior Biological Knowledge Improves Genomic Prediction of Growth-Related Traits in Arabidopsis thaliana. Frontiers in Genetics, 11:609117. doi: 10.3389/fgene.2020.609117
- He Y, Li Y, Yao Y et al. (2021) Overexpression of watermelon m6A methyltransferase ClMTB enhances drought tolerance in tobacco by mitigating oxidative stress and photosynthesis inhibition and modulating stress-responsive gene expression. Plant Physiology and Biochemistry, 168: 340-352.
- Wang W, Liu D, Qin M et al. (2021) Effects of Supplemental Lighting on Potassium Transport and Fruit Coloring of Tomatoes Grown in Hydroponics. International Journal of Molecular Sciences, 22(5): 2687 https://doi.org/10.3390/ijms22052687
- Singh R R, Pajar J A, Audenaert K, et al. (2021) Induced Resistance by Ascorbate Oxidation Involves Potentiating of the Phenylpropanoid Pathway and Improved Rice Tolerance to Parasitic Nematodes. Frontiers in Plant Science, 12:713870. doi: 10.3389/fpls.2021.713870
- Vidak M, Lazarevic B, Petek M, et al. (2021) Multispectral Assessment of Sweet Pepper (Capsicum annuum L.) Fruit Quality Affected by Calcite Nanoparticles. Biomolecules, 11(6), 832; https://doi.org/10.3390/biom11060832
- Lazarevic B, Satovic Z, Nimac A, et al. (2021) Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Frontiers in Plant Science, 12:629441. doi: 10.3389/fpls.2021.629441
- Romero-Perez A, Ameye M, Audenaert K, et al. (2021) Overexpression of F-Box Nictaba Promotes Defense and Anthocyanin Accumulation in Arabidopsis thaliana After Pseudomonas syringae Infection. Frontiers in Plant Science, 12:692606. doi: 10.3389/fpls.2021.692606
- Meng L, Mestdagh H, Ameye M, et al. (2021) Phenotypic variation of Botrytis cinerea Isolates is influenced by spectral light quality. Frontiers in Plant Science, 11:1233. doi: 10.3389/fpls.2020.01233
- De Zutter N, Ameye M, Debode J, et al. (2021) Shifts in the rhizobiome during consecutive in planta enrichment for phosphate-solubilizing bacteria differentially affect maize P status. Microbial Biotechnology, doi:10.1111/1751-7915.13824
- Stambuk P, Sikuten I, Preiner D, et al. (2021) Screening of Croatian Native Grapevine Varieties for Susceptibility to Plasmopara viticola Using Leaf Disc Bioassay, Chlorophyll Fluorescence, and Multispectral Imaging. Plants, 10, 661. https://doi.org/10.3390/plants10040661
- Tan J, de Zutter N, de Saeger S, et al. (2021) Presence of the Weakly Pathogenic Fusarium poae in the Fusarium Head Blight Disease Complex Hampers Biocontrol and Chemical Control of the Virulent Fusarium graminearum Pathogen. Frontiers in Plant Science, https://doi.org/10.3389/fpls.2021.641890
- Flood P, Theeuwen T, Schneeberger K, Keizer P, Kruijer W, et al. (2020) Reciprocal cybrids reveal how organellar genomes affect plant phenotypes. Nature Plants, 10.1038/s41477-019-0575-9ff. ffhal-02392124v2f
- Velivelli S L S, Czymmek K J, Li H, Shaw J B, Buchko G W, Shah D M. (2020) Antifungal symbiotic peptide NCR044 exhibits unique structure and multifaceted mechanisms of action that confer plant protection. PNAS, DOI: 10.1073/pnas.2003526117
- Bhatnagar N, Pandey S. (2020) Heterotrimeric G-Protein Interactions Are Conserved Despite Regulatory Element Loss in Some Plants. Plant Physiology, DOI: https://doi.org/10.1104/pp.20.01309
- Venneman J, Vandermeersch L, Walgraeve C et al. (2020) Respiratory CO2 Combined With a Blend of Volatiles Emitted by Endophytic Serendipita Strains Strongly Stimulate Growth of Arabidopsis Implicating Auxin and Cytokinin Signaling. Frontiers in Plant Science, https://doi.org/10.3389/fpls.2020.544435
- Tan J, Ameye M, Landschoot S et al. (2020) At the scene of the crime: New insights into the role of weakly pathogenic members of the fusarium head blight disease complex. Molecular Plant Pathology, DOI: 10.1111/mpp.12996
- Prinzenberg A E, Campos-Dominguez L, Kruijer W, Harbinson J, Aarts M G M. (2020) Natural variation of photosynthetic efficiency in Arabidopsis thaliana accessions under low temperature conditions. Plant Cell & Environment, 1–14. https://doi.org/10.1111/pce.13811
- Zhang H, Chen Y, Niu Y, Zhang X, Zhao J, Sun L, Wang H, Xiao J, Wang X. (2020) Characterization and fine mapping of a leaf yellowing mutant in common wheat. Plant Growth Regulation, https://doi.org/10.1007/s10725-020-00633-0
- Jin X, Zarco-Tejada P, Schmidhalter U, Reynolds M P et al. (2020) High-throughput estimation of crop traits: A review of ground and aerial phenotyping platforms. IEEE Geoscience and Remote Sensing Magazine, DOI: 10.1109/MGRS.2020.2998816
- Sheng X-G, Branca F, Zhao Z-Q et al. (2020) Identification of Black Rot Resistance in a Wild Brassica Species and Its Potential Transferability to Cauliflower. Argonomy, 10: 1400. doi:10.3390/agronomy10091400
- Pennisi G, Blasioli S, Cellini A, Maia L, Crepaldi A, Braschi I, Gianquinto G. (2019). Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Frontiers in plant science, 10, 305. doi:10.3389/fpls.2019.00305
- Pennisi G, Orsini F, Blasioli S, Cellini A et al. (2019) Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Scientific Reports, 9, 14127
- Van Es S W, van der Auweraert E B, Silveira S R, Angenent G C, van Dijk A D J, Immink R G H. (2019) Comprehensive phenotyping reveals interactions and functions of Arabidopsis thaliana TCP genes in yield determination. The Plant Journal, doi: 10.1111/tpj.14326
- Köhl J, Goossen-van de Geijn H, Groenenboom-de Haas L, et al. (2019) Stepwise screening of candidate antagonists for biological control of Blumeria graminis f. sp. tritici. Biological Control, 136: 104008
- Mohd Nadzir M M, Vieira Lelis F M, Thapa B, Ali A, Visser R G F, van Heusden A W, van der Wolf J M. (2019) Development of an in vitro protocol to screen Clavibacter michiganensis subsp. michiganensis pathogenicity in different Solanum species. Plant Phathology, 68(1): 42-48
- Sall K, Dekkers B J W, Nonogaki M, Katsuragawa Y, Koyari R, Hendrix D, Willems L A J, Bentsink L, Nonogaki H. (2019) DELAY OF GERMINATION 1‐LIKE 4 acts as an inducer of seed reserve accumulation. The Plant Journal, 100: 7-19.
- Li H, Velivelli S L S, Shah D M. (2019) Antifungal Potency and Modes of Action of a Novel Olive Tree Defensin Against Closely Related Ascomycete Fungal Pathogens. Molecular Plant-Microbe Interactions. 32(12): 1646-1664.
- Prinzenberg A E, Viquez-Zamora M, Harbinson J, Lindhout P, van Heusden S. (2018) Chlorophyll fluorescence imaging reveals genetic variationand loci for a photosynthetic trait in diploid potato. Physiologia Plantarum, 164: 163-175.
- Van Rooijen R, Harbinson J, Aarts M G M. (2018) Photosynthetic response to increased irradiance correlates to variation in transcriptional response of lipid‐remodeling and heat‐shock genes. Plant Direct, 2(7): e00069
- Van Bezouw R F H M, Keurentjes J J B, Harbinson J, Aarts M G. (2018) Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. Plant Journal, 97(1): 112-133.
- Domazakis E, Wouters D, Visser R G F, Kamoun S, Joosten M H A J, Vleeshouwers V G A A. (2018) The ELR-SOBIR1 Complex Functions as a Two-Component Receptor-Like Kinase to Mount Defense Against Phytophthora infestans. Molecular Plant-Microbe Interactions, 31(8): 795-802.
- Bazakos C, Hanemian M, Trontin C, Jimenez-Gomez J M, Loudet O. (2017) New Strategies and Tools in Quantitative Genetics: How to Go from the Phenotype to the Genotype. Annual Review of Plant Biology, 68:435-455
- Van Rooijen R, Kruijer W, Boesten R, van Eeuwijk F A, Harbinson J, Aarts M G M. (2017) Natural variation of YELLOW SEEDLING1 affects photosynthetic acclimation of Arabidopsis thaliana. Nature Communications, 8: 1421
- Flood P J, Kruijer W, Schnabel S K, van der Schoor R, Jalink H, Snel J F H, Harbinson J, Aarts M G M. (2016) Phenomics for photosynthesis, growth and reflectance in Arabidopsis thaliana reveals circadian and long-term fluctuations in heritability. Plant Methods, 12: 14. https://doi.org/10.1186/s13007-016-0113-y
- Mancarella S, Orsini F, van Oosten M J, SAnoubar R, Stanghellini C, Kondo S, Gianquinto G, Maggio A. (2016) Leaf sodium accumulation facilitates salt stress adaptation and preserves photosystem functionality in salt stressed Ocimum basilicum. Environmental and Experimental Botany, 130: 162-173.
- Virlet N, Sabermanesh K, Sadeghi-Tehran P, Hawkesford M J. (2016) Field Scanalyzer: An automated robotic field phenotyping platform for detailed crop monitoring. Functional Plant Biology, 44(1): 143-153.
- Gorbe Sanchez E, Heuvelink E, de Gelder A, Stanghellini C. (2015) New Non-invasive Tools for Early Plant Stress Detection. Procedia Environmental Sciences, 29: 249-250.
- Kastelein P, Krijger M, Czajkowski R, van der Zouwen P S, van der Schoor R, Jalink H, van der Wolf J M. (2014) Development of Xanthomonas fragariae populations and disease progression in strawberry plants after spray‐inoculation of leaves. Plant Pathology, 63(2): 255-263.
- Harbinson J, Prinzenberg A E, Kruijer W, Aarts M G M. (2012) High throughput screening with chlorophyll fluorescence imaging and its use in crop improvement. Current Opinion in Biotechnology, 23:221
案例与文献
2021-03-05
2021-02-24
推荐新闻
本期石时之约,我们将对话慧诺瑞德(北京)科技有限公司总经理、国际植物表型学会(IPPN)执委会委员/工业分会副主席韩志国,一起从表型数据的科学角度,去读懂农作物的喜怒哀乐和前世今生。
让我们“慧聚”在一起,为“慧科研、慧育种、慧种田”赋能。
育种,是在给定的环境条件下,选择各种表型指标(产量、品质、抗性)最优的基因型材料的过程(AI育种,从这里起步)。育种工作中大约70%的工作量来自表型观察测量和筛选,是最耗人力物力的过程。
生理表型测量的核心在于“早、快”,要在肉眼可见之前就能测量并预判出变化趋势,才是这个技术的核心价值。叶绿素荧光成像,恰好满足了这个要求。
视频展示
